Introduction
The landscape of hematology research is rapidly advancing with promising data emerging from clinical trials across sickle cell disease, acute myeloid leukemia (AML) and chronic leukemia. Recent reports from Clinical Trial Vanguard highlight durable treatment profiles, high response rates and the launch of a pivotal Phase III study.
Below are three key developments that reflect major progress in blood disorder therapeutics.
1. BEAM’s Risto-Cel Shows Durable Profile in Sickle Cell Trial
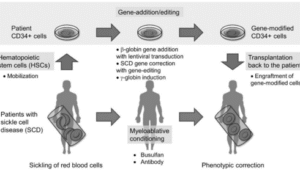
In sickle cell disease research, BEAM Therapeutics’ investigational therapy risto-cel has demonstrated a durable safety and response profile in early clinical evaluation.
🔗 Read more: BEAM’s Risto-Cel Shows Durable Profile in Sickle Cell Trial
Sickle cell disease is a genetic disorder characterized by painful vaso-occlusive episodes, chronic hemolysis and multi-organ complications. Early clinical results from risto-cel suggest that the therapy’s mechanism may provide sustained clinical benefit with a manageable safety profile, offering hope for patients where therapeutic options remain limited.
The durability of response seen in this study underscores the potential of gene-editing platforms to transform long-term disease management.
2. Tuspetinib Triple Therapy Shows High AML Response Rate at ASH
At the American Society of Hematology (ASH) annual meeting, updated data for tuspetinib triple therapy revealed a high response rate in patients with acute myeloid leukemia (AML).
🔗 Read more: Tuspetinib Triple Therapy Shows High AML Response Rate at ASH
AML is an aggressive blood cancer with historically poor outcomes. The latest clinical results from the ASH presentation indicate that combining tuspetinib with other therapies may significantly enhance response rates in this challenging patient population. High response rates in early clinical settings provide strong support for continued evaluation and optimization of this regimen.
These findings contribute to a growing body of evidence that rational combinations can improve outcomes in blood cancers that have been resistant to single-agent therapies.
3. Quetzal Launches Phase III Trial for Leukemia Drug QTX-2101
In another important development, Quetzal Bio has initiated a Phase III clinical trial to evaluate its investigational leukemia therapy QTX-2101.
🔗 Read more: Quetzal Launches Phase III Trial for Leukemia Drug QTX-2101
Phase III trials represent a pivotal step toward potential regulatory approval and broader clinical use. QTX-2101’s progression into this stage reflects encouraging earlier data and confidence in its ability to provide meaningful clinical benefit to patients with specific forms of leukemia.
The launch of a Phase III study underscores the continued investment and clinical commitment to advancing novel leukemia therapies from bench to bedside.
Conclusion
From durable clinical profiles in sickle cell disease to high AML response rates and the launch of a Phase III leukemia trial, these updates exemplify how innovative science and careful clinical execution are bringing new therapeutic possibilities to patients with hematologic disorders.
For more detailed clinical insights and ongoing trial coverage, visit Clinical Trial Vanguard.
SEO Meta Information
Meta Title:
Hematology Research Advances: Sickle Cell, AML Response Data and Phase III Leukemia Trial
Meta Description:
Explore the latest hematology clinical trial updates, including durable risto-cel data in sickle cell, high response rates for tuspetinib triple therapy in AML, and Quetzal’s Phase III leukemia trial launch.
Keywords:
sickle cell disease, risto-cel, BEAM Therapeutics, AML response rate, tuspetinib, Phase III leukemia trial, QTX-2101, Quetzal Bio, Clinical Trial Vanguard
